Technology
Immunology
The immune system is composed of immune cells, tissues, and organs that work together to protect our body from pathogen infection, or to distinguish abnormal cells from normal cells.
Immune Cells
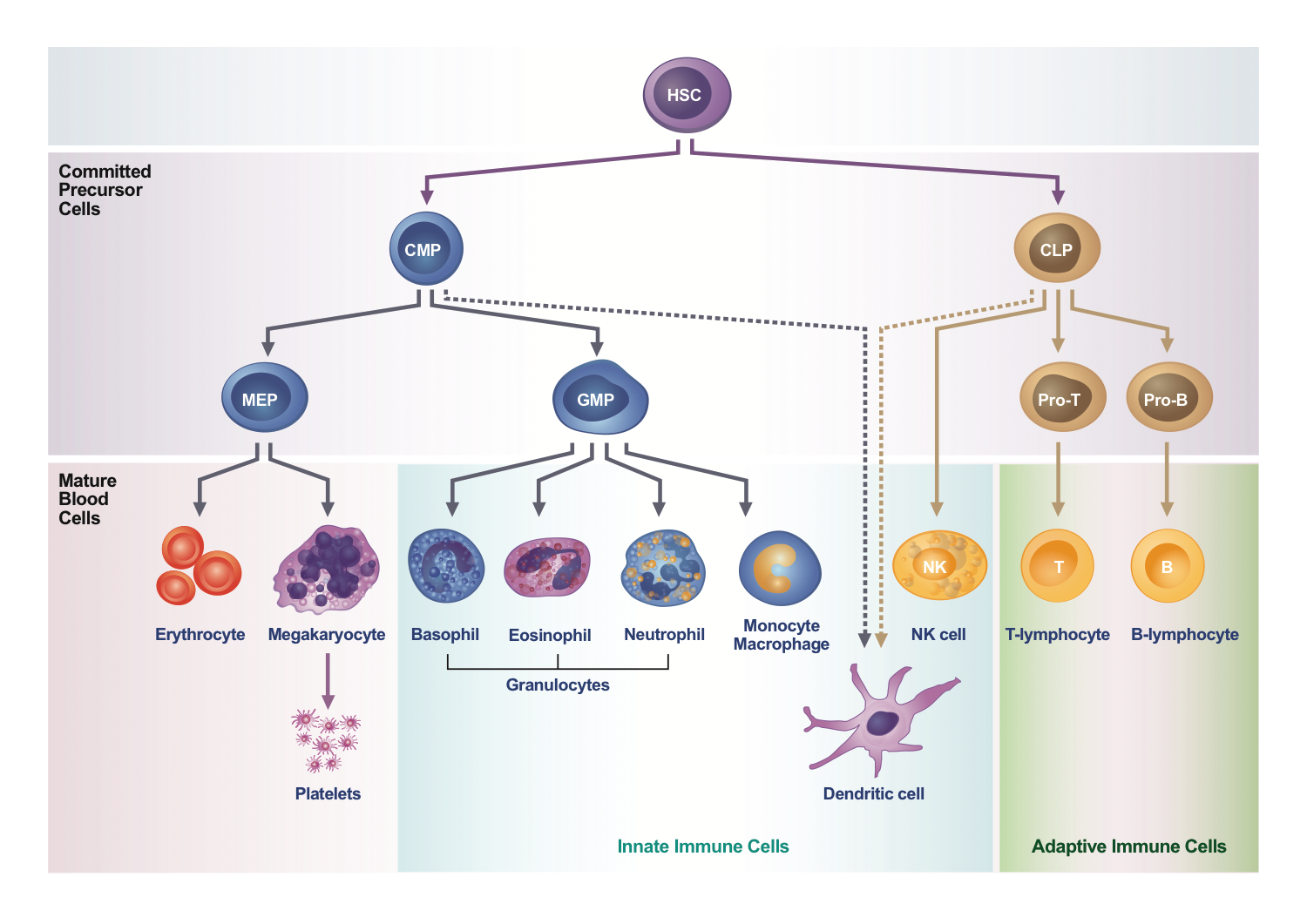 Generally, immune cells are divided into innate and adaptive immune cells. Innate immune cells contain granulocytes, mast cells, monocytes, dendritic cells, and natural killer (NK) cell. When innate immune cells recognize foreign antigen or abnormal cells, they immediately generate non-specific immune response by uptaking pathogens or killing the cells, and then activate adaptive immune cells by releasing cytokines or presenting antigen on cell surface. Adaptive immune cells, including T cells and B cells, proliferate a lot and mediate antigen-specific immune response after activation. Adaptive immune cells also could generate immunological memory later.
Generally, immune cells are divided into innate and adaptive immune cells. Innate immune cells contain granulocytes, mast cells, monocytes, dendritic cells, and natural killer (NK) cell. When innate immune cells recognize foreign antigen or abnormal cells, they immediately generate non-specific immune response by uptaking pathogens or killing the cells, and then activate adaptive immune cells by releasing cytokines or presenting antigen on cell surface. Adaptive immune cells, including T cells and B cells, proliferate a lot and mediate antigen-specific immune response after activation. Adaptive immune cells also could generate immunological memory later.
Cell-Based Immunotherapy
 Traditionally, surgery, chemotherapy and radiation therapy have been used as primary strategies to treat tumors in patients. These therapies can eliminate cancer cells or give patients some relief; however, remaining cancer cells or cancer cells which become resistant to drug treatment might lead to an increased incidence of tumor relapse.
Traditionally, surgery, chemotherapy and radiation therapy have been used as primary strategies to treat tumors in patients. These therapies can eliminate cancer cells or give patients some relief; however, remaining cancer cells or cancer cells which become resistant to drug treatment might lead to an increased incidence of tumor relapse.
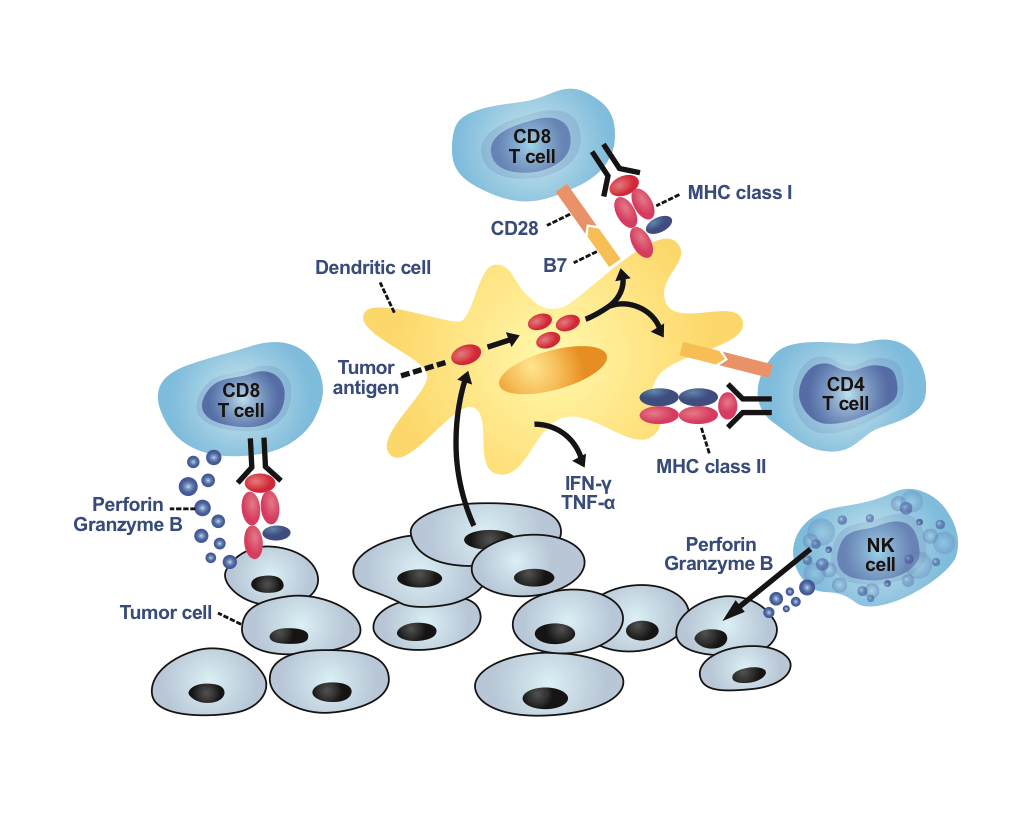
More effective therapies are needed, and are continually being developed by scientists. Cell-based immunotherapy is a gentle and adjuvant treatment that uses immune cells to destroy cancer cells. It could be combined with traditional therapies to achieve better curative effects in treating cancer patients. The cells types used most frequently in clinical cases include NK cells, dendritic cells, and T cells.
- NK cells: NK cells belong to the member of innate immune cells. When NK cells recognize abnormal cells, including cancer cells and virus-infected cells, they directly kill abnormal cells through three mechanisms: cytoplasmic granule release, antibody-dependent cell-mediated cytotoxicity (ADCC), and death receptor-induced apoptosis.
- Dendritic cells: Dendritic cells are the most powerfully professional antigen-processing cells. Dendritic cells uptake apoptotic tumor cells, process the antigen material, and then present it on the surface to the T cells. Dendritic cells play an important role in activating tumor antigen-specific T cell response.
- T cells:cells: T cells are the member of adaptive immune cells, and are generally divided into three groups: cytotoxic, helper and regulatory T cells. Cytotoxic T cells are the most effective cell type for eliminating cancer cells through releasing cytoplasmic granule and inducing death receptor-mediated apoptosis.
Adoptive Cell Therapy
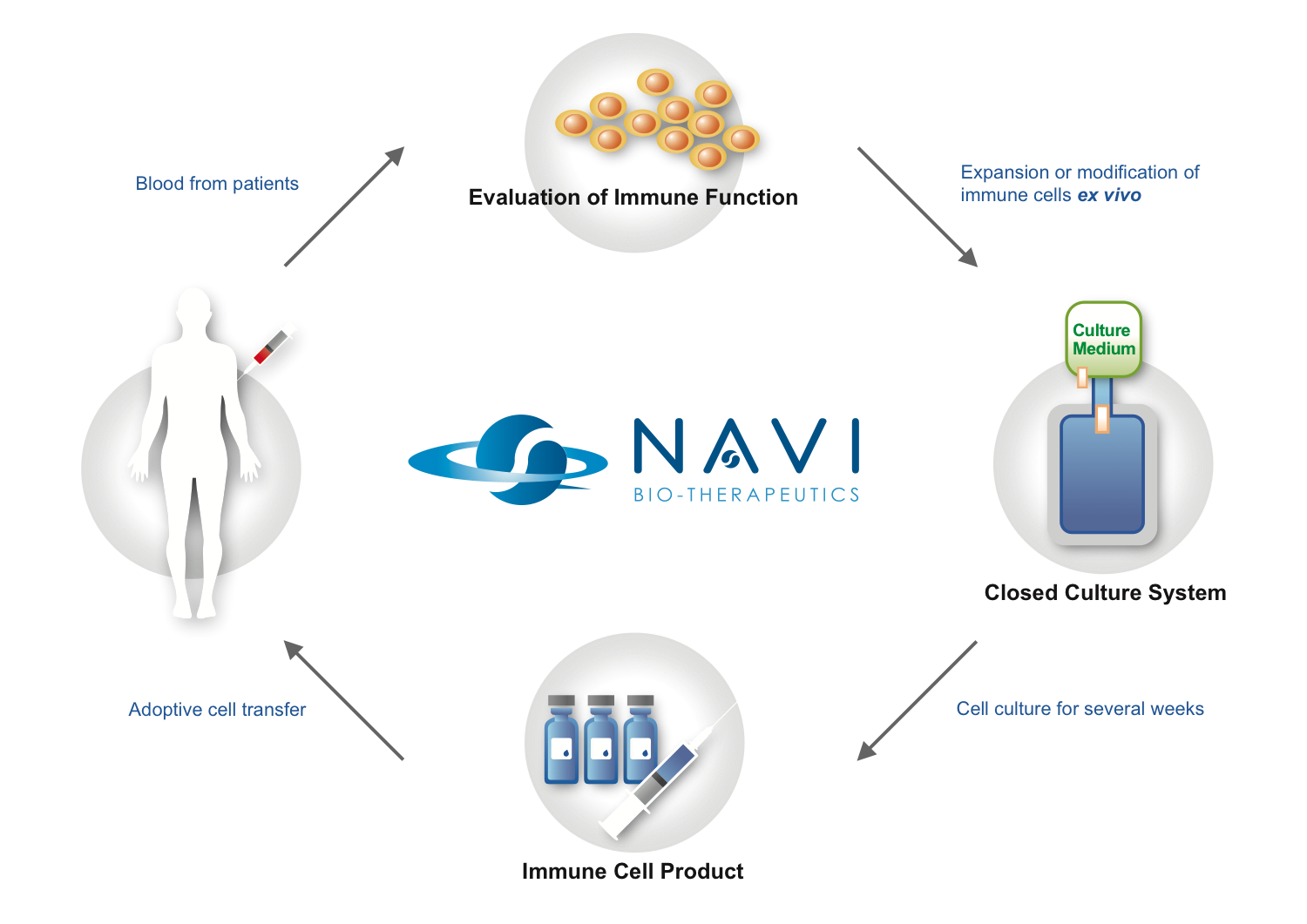 Adoptive Cell Therapy (ACT) is a treatment in which immune cells are isolated from the patient, expanded and/or modified ex vivo, and then transferred back to the patients to recognize and kill tumor cells. There are many forms of ACT used in clinical cancer treatment including tumor infiltrating lymphocytes (TILs), cytotoxic T cells, NK cells, dendritic cells, lymphokine-activated killer (LAK) cells, cytokine-induced killer (CIK) cells, TCR (T cell receptor)- or CAR (chimeric antigen receptor)-engineered T cells. Several clinical studies have demonstrated that ACT is feasible and effective in cancer treatment. NAVI is devoted to the development of ex vivo manipulation techniques of immune cells, and provides our partners with technical service.
Adoptive Cell Therapy (ACT) is a treatment in which immune cells are isolated from the patient, expanded and/or modified ex vivo, and then transferred back to the patients to recognize and kill tumor cells. There are many forms of ACT used in clinical cancer treatment including tumor infiltrating lymphocytes (TILs), cytotoxic T cells, NK cells, dendritic cells, lymphokine-activated killer (LAK) cells, cytokine-induced killer (CIK) cells, TCR (T cell receptor)- or CAR (chimeric antigen receptor)-engineered T cells. Several clinical studies have demonstrated that ACT is feasible and effective in cancer treatment. NAVI is devoted to the development of ex vivo manipulation techniques of immune cells, and provides our partners with technical service.
Research
 NAVI focused on developing cell-based immunotherapy for cancer.
NAVI focused on developing cell-based immunotherapy for cancer.
- Cell-based immunotherapy: NAVI expects to advance our therapeutic platform of NK cells to a clinical trial in cooperation with National Taiwan University Hospital in 2016. NK cells are generated and expanded ex vivo. When reintroduced back to patients, activated NK cells can recognize and kill residual tumor cells. Adjuvant NK cell therapy is anticipated to prolong the overall survival and progression-free survival rates of cancer patients, accompanied by an improved quality of life.
- Autologous Multi-Immune Cell Therapy (MICT): MICT is a kind of adoptive cell therapy that combines antigen-presenting ability of dendritic cells with tumor-killing ability of cytokine-induced killer cells. MICT relies on the crosstalk between immune cells to induce better active immune response to tumor cells.
- Genetically engineered immune cells: Chimeric antigen receptors (CARs) are genetically expressed on the cell surface of T cells or NK cells. CARs, composed of a single-chain variable fragment (scFv), T cell receptor CD3zeta chain, and a co-stimulatory molecular (such as CD28 or 4-1BB), are the proteins that allow the engineered cells to recognize a specific antigen on tumor cells. Upon antigen recognition, CARs could deliver an activating signaling in engineered immune cells, leading the killing of target tumor cells.
- Gene therapy: Application of siRNA in cancer treatment
- Closed culture system for the separation and expansion of immune cells: Manufacture of cell products not only needs to maintain the cell activity but also to prevent contamination from microorganism. The method by which cells were separated and expanded in a closed culture system highly reduces the contamination risk.
Reference
- Suen, J. L., Chuang, Y. H., Tsai, B. Y., Yau, P. M., & Chiang, B. L. (2004). Treatment of murine lupus using nucleosomal T cell epitopes identified by bone marrow-derived dendritic cells. Arthritis & Rheumatism, 50(11), 3250–3259.
- Tsai, B. Y., Lin, Y. L., & Chiang, B. L. (2009). Application of interleukin-12 expressing dendritic cells for the treatment of animal models of leukemia. Experimental Biology and Medicine (Maywood), 234(8), 952–960.
- Tsai, B. Y., Lin, Y. L., & Chiang, B. L. (2010). Autoimmune response induced by dendritic cells exerts anti-tumor effect in murine model of leukemia. Journal of Autoimmunity, 34(4), 364–370.
- Tsai, B. Y., Suen, J. L., & Chiang, B. L. (2010). Lentiviral-mediated Foxp3 RNAi suppresses tumor growth of regulatory T cell-like leukemia in a murine tumor model. Gene Therapy, 17(8), 972–979.
- Rosenberg, S. A., & Restifo, N. P. (2015). Adoptive cell transfer as personalized immunotherapy for human cancer. Science, 348(6230), 62–68.
- Schmeel, L. C., Schmeel, F. C., Coch, C., & Schmidt-Wolf, I. G. (2015). Cytokine-induced killer (CIK) cells in cancer immunotherapy: Report of the international registry on CIK cells (IRCC). Journal of Cancer Research and Clinical Oncology, 141(5), 839–849.
- Childs, R. W., & Carlsten, M. (2015). Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: The force awakens. Nature Reviews Drug Discovery, 14(7), 487–498.
- Dahlberg, C. I., Sarhan, D., Chrobok, M., Duru, A. D., & Alici, E. (2015). Natural killer cell-based therapies targeting cancer: Possible strategies to gain and sustain anti-tumor activity. Frontiers in Immunology, 6, 605.